(GOX) is an important oxidoreductase enzyme with many important roles in biological processes. It is considered an “ideal enzyme” and is often called an oxidase “Ferrari” because of its fast mechanism of action, high stability and specificity. Glucose oxidase catalyzes the oxidation of 𝛽-d-glucose to d-glucono-𝛿-lactone and hydrogen peroxide in the presence of molecular oxygen. d-glucono-𝛿-lactone is sequentially hydrolyzed by lactonase to d-gluconic acid, and the resulting hydrogen peroxide is hydrolyzed by catalase to oxygen and water. GOX is presently known to be produced only by fungi and insects. The current main industrial producers of glucose oxidase are Aspergillus and Penicillium. An important property of GOX is its antimicrobial effect against various pathogens and its use in many industrial and medical areas. The aim of this review is to summarize the structure, function, production strains and biophysical and biochemical properties of GOX in light of its various industrial, biotechnological and medical applications.
Glucose oxidase is an enzyme that has widespread applications in industry and biotechnology. Due to this, a deep understanding of its structure and function are warranted. Glucose degradation is the most universal metabolic process. In addition to its breakdown in glycolysis, glucose can also be directly oxidized to glucono-𝛿-lactone by a number of enzymes.
These fall into two classes: the dehydrogenases glucose dehydrogenase (𝛽-d-glucose: NAD(P)+ 1-oxidoreductase, E.C. 1.1.1.47) and quinoprotein glucose dehydrogenase (d-glucose:ubiquinone oxidoreductase, E.C. 1.1.5.2) andthe oxidases glucose oxidase (GOX; 𝛽-d-glucose:oxygen 1-oxidoreductase, E.C. 1.1.3.4) and pyranose oxidase (pyranose:oxygen 2-oxidoreductase, E.C. 1.1.3.10).
The dehydrogenases oxidize glucose in one step using a co-factor, either nicotinamide adenine dinucleotide (phosphate) (NAD(P)+) or pyrroloquinoline quinone (PQQ), as the electron sink while the oxidases use a two-step mechanism in which a bound flavin adenine dinucleotide (FAD) co-factor is used to oxidize glucose to form glucono-𝛿-lactone and an enzyme-FADH2 intermediate followed by electron transfer to O2 to form H2O2. The principal difference between GOX and pyranose oxidase is that the former is specific to 𝛽-d-glucose while the latter is also able to act on d-xylose, l-sorbose and d-galactose.
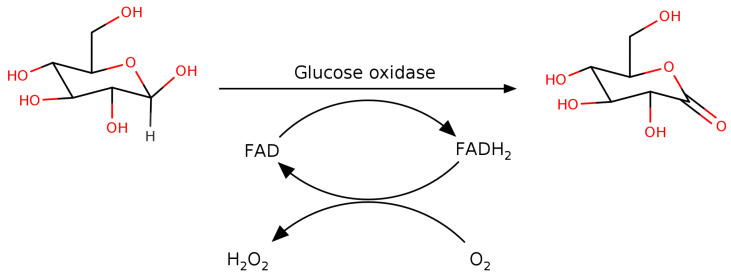
The general reaction of GOX
The high specificity, high turnover and high stability of GOX make it an ideal enzyme for biosensor applications, some of which will be described below. Although its rate constant is still several orders of magnitude below the diffusion limit, GOX has a much higher 𝑘cat/𝐾M (on the order of 106M−1·s−1) compared with most other oxidoreductases, prompting at least one researcher to call it “the Ferrari of the oxidases” .
GOX is a member of the glucose-methanol-choline oxidoreductase (GMC oxidoreductase) superfamily. The members of this family are all FAD-dependent oxidoreductases that share a common fold . They consist of two functional domains, an N-terminal FAD-binding domain, which contains a strictly conserved 𝛽𝛼𝛽 mononucleotide-binding motif and a more variable substrate binding-domain. As the name suggests, the members of this family oxidize a variety of substrates containing hydroxyl functional groups, including mono and di-saccharides, alcohols, cholesterol and choline. GOX is perhaps the most thoroughly characterized of these, and its mechanism will be described more thoroughly below.
GOX is used in many branches of industry because of its ability to oxidize glucose and produce hydrogen peroxide. Its rapid turnover and high stability finds it many applications in the food, pharmaceutical, medical, textile and power industries. For many of these applications GOX is used in a biosensor or nanosensor , in nanoparticles or in nanosheets .
In many modern applications, GOX is often used in combination with other enzymes, for example, tyrosinase in the analysis and discrimination of musts and wines, 𝛼-amylases and xylanases for improving the quality of dough and bread, peroxidase for accurately measuring the level of glucose in blood and saliva and tears, the autophagy inhibitor chloroquine in cancer intervention therapy and insulin for regulating blood glucose levels in diabetes. Finally, it has been combined with the anti-cancer drug tirapazamine and human serum albumin to create a nanoreactor capable of increasing the levels of hypoxia and reactive oxygen species and inhibiting tumor growth.
The applicability of GOX primarily depends on its quantity, thermal stability and activity. Many studies focused on identifying which fungal strains are better for biosensor development, which are better for clinical studies and which are better for biochemical diagnostic tests. Optimal GOX utilization also requires consideration of the type of matrix on which GOX is bound and type of media and conditions under which it is used. Consequently, in addition to identifying the best GOX producers and the ideal conditions for its stability and activity, the development of different binding materials, environmental conditions and detection systems are also very important for expanding the range of GOX’s industrial applications.
Quote : https://pmc.ncbi.nlm.nih.gov/articles/PMC8946809/#sec5-biomolecules-12-00472

